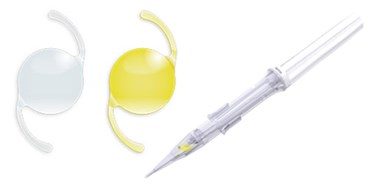
The glistening-free hydrophobic Vivinex™ IOL preloaded in the proven Vivinex™ iSertⓇ injector provides unprecedented clarity of vision.
Vivinex™ iSert™
UNPRECEDENTED CLARITY OF VISION.
The preloaded Vivinex™ IOL provides unprecedented clarity of vision in the proven
Vivinex™ iSert® injector
Designed to provide outstanding optical quality, Vivinex™ offers unprecedented clarity of vision for patients suffering from cataract. Product quality, trust, dedication and attention to detail are deeply rooted in our Japanese heritage and with 1 million lenses sold worldwide, surgeons‘ trust in Vivinex™ is proven.
- Glistening-free hydrophobic acrylic IOL material 1,3
- Proprietary aspheric optic design for improved image quality 5
- Active oxygen processing treatment and sharp optic edge to reduce PCO 1
1 Clinical Evaluation of the HOYA VivinexTM IOL, HOYA data on file DoF-PHIV-101-SP2-12mIR-31082018 (2018).
3 Glistening-free per Miyata scale; study result of the David J Apple International Laboratory for Ocular Pathology, University Hospital Heidelberg. Report on file.
5 Pérez-Merino P, Marcos S. Effect of intraocular lens decentration on image quality tested in a custom model eye. J Cataract Refract Surg. 2018;44(7):889–896.
Specifications:
| Model Names | Vivinex™ iSertⓇ XC1, Vivinex™ iSertⓇ XY1 |
| Optic Material | Hydrophobic acrylic Vivinex™ with UV-Filter (Model XC1) with UV- and blue light filter (Model XY1) |
| Optic Design | Aspheric Design with sharp textured optic edge |
| Haptic Design | Textured-rough haptic surface |
| Haptic Material | Hydrophobic acrylic Vivinex™ with UV-Filter (Model XC1) with UV- and blue light filter (Model XY1) |
| Dimension (Optic/OAL) | 6.00 mm / 13.00 mm |
| Power | +6.00 to +30.00 D (in 0.50 D increments) |
| A-Constant | 118.9 |
| Nominal A-Constant | 118.9 |
| Optimized Optical constants |
Haigis a0 = -0.278 a1 = 0.215 a2 = 0.201 |
| Front injector tip outer diameter | 1.70 mm (Vivinex™ iSertⓇ preloaded) |
Available in Saudi Arabia, UAE, Oman, Qatar, Kuwait and Lebanon
ivinex™ Toric
UNPRECEDENTED CLARITY AND ROTATIONAL STABILITY.
Designed for outstanding optical quality, Vivinex™ Toric provides proven rotational stability for precise astigmatism correction and unprecedented clarity of vision for cataract patients. Product quality, trust, dedication and attention to detail are deeply rooted in our japanese heritage.
Vivinex™ Toric offers the following benefits:
- Glistening-free hydrophobic acrylic IOL material1,3
- Proprietary aspheric optic design for improved image quality2
- Active oxygen processing treatment and sharp optic edge to reduce PCO3
- Vivinex™ Toric IOL preloaded in the proven VivinexTM iSert® injector system
- Outstanding rotational stability4
- Median rotation 1.1° [ range: 0.0° – 5.0° ]4
- 100% of lenses (n=103) had 5° of rotation from their initial axis at end of surgery through all follow up visits at 1 hour, 1 week and 6 months4
2 Pérez-Merino P, Marcos S. Effect of intraocular lens decentration on image quality tested in a custom model eye. J Cataract Refract Surg. 2018;44(7):889–896.
3 Data on File of Study PHIV-101-SP2: Clinical Evaluation of the HOYA Vivinex IOL (2018).
4 Schartmüller D, Schriefl S, Schwarzenbacher L, Leydolt C,Menapace R. True rotational stability of a single-piece hydrophobic intraocular lens. Br J Ophthalmol. 2018 Apr 17. pii: bjophthalmol-2017-311797. doi: 10.1136/ bjophthalmol-2017-311797.
| Model Names | Vivinex™ Toric XY1A |
| Optic Material | Hydrophobic acrylic Vivinex™ with UV- and blue light filter |
| Optic Design | Biconvex with sharp textured optic edge Anterior: Aspheric Design Posterior: Toric Design |
| Haptic Design | Textured-rough haptic surface |
| Haptic Material | Hydrophobic acrylic Vivinex™ with UV- and blue light filter |
| Dimension (Optic/OAL) | 6.00 mm / 13.00 mm |
| Power | +10.00 to +30.00 D (in 0.50 D increments) |
| Cylindrical Power | 1.00 to 6.00 D (T2 to T9) T2 to T3 in 0.50 D increments T3 to T9 in 0.75 D increments |
| A-Constant | 118.9 |
| Optimized Optical constants |
Haigis a0 = -0.278 a1 = 0.215 a2 =0.201 |
| Front injector tip outer diameter | 1.70 mm (Vivinex™ iSert® preloaded) |

Designed to provide outstanding optical quality, Vivinex™ offers clarity of vision for patients suffering from cataract. Vivinex™ is made from a novel glistening-free hydrophobic acrylic, using a proprietary manufacturing process that includes a unique, active oxygen posterior surface treatment [1,2,3]. This as well as its square edge design and one of the smoothest and most regular IOL surfaces has been shown to provide a low incidence of PCO in several studies [2,4,5,6,7,8,9,10].
multiSert™ provides unmatched control at the fingertips; it allows single-handed push and two-handed screw injection within one device. Has a uniquely designed adjustable insert shield for precise injector tip insertion depth management, allowing to insert the injector tip either directly into the capsular bag or through the incision wound tunnel. Moreover, multiSert™ provides outstandingly consistent and predictable IOL delivery [11].
Specifications
|
Model name |
XC1-SP / XY1-SP |
|
Optic material |
Hydrophobic acrylic Vivinex™ with UV-Filter (Model XC1-SP) with UV- and blue light filter (Model XY1-SP) |
|
Optic design |
Aspheric Design with square, thin and textured optic edge |
|
Haptic design |
Textured-rough haptic surface |
|
Haptic material |
Hydrophobic acrylic Vivinex™ with UV-Filter (Model XC1-SP) with UV- and blue light filter (Model XY1-SP) |
|
Diameter (optic/OAL) |
6.00 mm / 13.00 mm |
|
Power |
+6.00 to +30.00 D (in 0.50 D increments) |
|
Injector |
multiSert™ preloaded |
|
Front injector tip outer diameter |
1.70mm |
|
Recommended incision size |
2.20mm |
|
Nominal A-constant * |
118.9 |
|
Optimized constants ** |
Haigis a0 = -0.8028 a1 = 0.2133 a2 = 0.2245 |
* The A-constant is presented as a starting point for the lens power calculation. When calculating the exact lens power, it is recommended that calculations be performed individually, based on the equipment used and operating surgeon’s own experience
** These optimized constants for the calculation of intraocular lens power published by IOLCon on their website: https://iolcon.org are calculated from 1,475 clinical results for Vivinex™ model XY1/XC1 as of September 24, 2021. These constants are based on actual surgical data and are provided by IOLCon as a starting point for individual constant optimizations. The information available on the website is based on data originating from other users and not by HOYA Surgical Optics (“HSO”). HSO therefore does not warrant the correctness, completeness and currentness of the contents on the said website.
Some of the products and/or specific features may not be registered in your country and thus may not be available there.
References
1. Glistening-free per Miyata scale; study result of the The David J Apple International Laboratory for Ocular Pathology, University Hospital Heidelberg. Report on file.
2. HOYA data on file. DoF-CTM-21-002, HOYA Medical Singapore Pte. Ltd, 2021
3. Pérez-Merino, P.; Marcos, S. (2018): Effect of intraocular lens decentration on image quality tested in a custom model eye. In: Journal of cataract and refractive surgery 44 (7), p. 889–896
4. Leydolt, C. et al. (2020): Posterior capsule opacification with two hydrophobic acrylic intraocular lenses: 3-year results of a randomized trial. In: American journal of ophthalmology 217 (9), p. 224-231
5. Giacinto, C. et al. (2019): Surface properties of commercially available hydrophobic acrylic intraocular lenses: Comparative study. In: Journal of cataract and refractive surgery 45 (9), p. 1330–1334.
6. Werner, L. et al. (2019): Evaluation of clarity characteristics in a new hydrophobic acrylic IOL in comparison to commercially available IOLs. In: Journal of cataract and refractive surgery 45 (10), p. 1490–1497
7. Matsushima, H. et al. (2006): Active oxygen processing for acrylic intraocular lenses to prevent posterior capsule opacification. In: Journal of cataract and refractive surgery 32 (6), p. 1035–1040.
8. Farukhi, A. et al. (2015): Evaluation of uveal and capsule biocompatibility of a single-piece hydrophobic acrylic intraocular lens with ultraviolet-ozone treatment on the posterior surface. In: Journal of cataract and refractive surgery 41 (5), p. 1081–1087.
9. Eldred, J. et al. (2019): An In Vitro Human Lens Capsular Bag Model Adopting a Graded Culture Regime to Assess Putative Impact of IOLs on PCO Formation. In: Investigative ophthalmology & visual science 60(1), p. 113–122.
10. Nanavaty, M. et al. (2019): Edge profile of commercially available square-edged intraocular lenses: Part 2. In: Journal of cataract and refractive surgery 45 (6), p. 847–853
11. Usability and acceptability evaluation of the multiSert™ injector system, HOYA data on file DoF-SERT-102-MULT-03052018 (2018).
Designed to provide precise astigmatism correction and clarity of vision for patients with an astigmatic cornea. Vivinex™ Toric IOL offers outstanding optical quality and rotational stability, delivered by multiSert™, providing unmatched control at your fingertips [3,10,11].
Vivinex™ Toric, made of a glistening-free hydrophobic acrylic IOL material [1,2] with proprietary aspheric optic design for improved image quality [3], allows active oxygen processing treatment, a smooth surface and square optic edge to reduce PCO [2,4,5,6,7,8,9,10], as well as Median rotation 1.1° [range: 0.0°–5.0°] 100% of lenses (n=103) had ≤5° of rotation from their initial axis at end of surgery through all follow up visits at 1 hour, 1 week, 1 month and 6 months [11].
Specifications
|
Model name |
XY1A-SP |
|
Optic design |
Biconvex with square, thin and textured optic edge |
|
Optic material |
Hydrophobic acrylic Vivinex™ with UV- and blue light filter |
|
Haptic design |
Textured-rough haptic surface |
|
Haptic material |
Hydrophobic acrylic Vivinex™ with UV- and blue light filter |
|
Diameter (optic/OAL) |
6.00 mm / 13.00 mm |
|
Power |
+10.00 to +30.00 D (in 0.50 D increments) |
|
Cylindrical Power |
1.00 to 6.00 D (T2 to T9), T2 to T3 in 0.50 D increments, T3 to T9 in 0.75 D increments |
|
Injector |
multiSert™ preloaded |
|
Front injector tip outer diameter |
1.70mm |
|
Recommended incision size |
2.20mm |
|
Nominal A-constant * |
118.9 |
|
Optimized constants ** |
Haigis a0 = -0.8028 a1 = 0.2133 a2 = 0.2245 |
|
Model XY1A-SP |
Cylinder Power |
Cylinder Power |
|
T2 |
1.00 D |
0.69 D |
|
T3 |
1.50 D |
1.04 D |
|
T4 |
2.25 D |
1.56 D |
|
T5 |
3.00 D |
2.08 D |
|
T6 |
3.75 D |
2.60 D |
|
T7 |
4.50 D |
3.12 D |
|
T8 |
5.25 D |
3.64 D |
|
T9 |
6.00 D |
4.17 D |
* The A-constant is presented as a starting point for the lens power calculation. When calculating the exact lens power, it is recommended that calculations be performed individually, based on the equipment used and operating surgeon’s own experience.
** These optimized constants for the calculation of intraocular lens power published by IOLCon on their website: https://iolcon.org are calculated from 1,475 clinical results for Vivinex™ Model XY1/XC1 as of September 17, 2021. These constants are based on actual surgical data and are provided by IOLCon as a starting point for individual constant optimizations. The information available on the website is based on data originating from other users and not by HOYA Surgical Optics (“HSO”). HSO therefore does not warrant the correctness, completeness and currentness of the contents on the said website.
*** Based on an average pseudophakic human eye.
Some of the products and/or specific features may not be registered in your country and thus may not be available there.
References
1 Glistening-free per Miyata scale; study result of the David J Apple International Laboratory for Ocular Pathology, University Hospital Heidelberg. Report on file.
2 HOYA data on file. DoF-CTM-21-002, HOYA Medical Singapore Pte. Ltd, 2021.
3 Pérez-Merino, P.; Marcos, S. (2018): Effect of intraocular lens decentration on image quality tested in a custom model eye. In: Journal of cataract and refractive surgery 44 (7), p. 889–896.
4 Leydolt, C. et al. (2020): Posterior capsule opacification with two hydrophobic acrylic intraocular lenses: 3-year results of a randomized trial. In: American journal of ophthalmology 217 (9), p. 224-231.
5 Giacinto, C. et al. (2019): Surface properties of commercially available hydrophobic acrylic intraocular lenses: Comparative study. In: Journal of cataract and refractive surgery 45 (9), p. 1330–1334.
6 Werner, L. et al. (2019): Evaluation of clarity characteristics in a new hydrophobic acrylic IOL in comparison to commercially available IOLs. In: Journal of cataract and refractive surgery 45 (10), p. 1490–1497.
7 Nanavaty, M. et al. (2019): Edge profile of commercially available square-edged intraocular lenses: Part 2. In: Journal of cataract and refractive surgery 45 (6), p. 847–853.
8 Matsushima, H. et al. (2006): Active oxygen processing for acrylic intraocular lenses to prevent posterior capsule opacification. In: Journal of cataract and refractive surgery 32 (6), p. 1035–1040.
9 Farukhi, A. et al. (2015): Evaluation of uveal and capsule biocompatibility of a single-piece hydrophobic acrylic intraocular lens with ultraviolet-ozone treatment on the posterior surface. In: Journal of cataract and refractive surgery 41 (5), p. 1081–1087.
10 Eldred, J. et al. (2019): An In Vitro Human Lens Capsular Bag Model Adopting a Graded Culture Regime to Assess Putative Impact of IOLs on PCO Formation. In: Investigative ophthalmology & visual science 60 (1), p. 113–122.
11 Schartmüller, D. et al. (2019): True rotational stability of a single-piece hydrophobic intraocular lens. In: The British journal of ophthalmology 103 (2), p. 186–190.
12 HOYA data on file. DoF-SERT-102-MULT-03052018, HOYA Medical Singapore Pte. Ltd, 2018 13 At IOL plane. 14 Based on an average pseudophakic human eye.
Vivinex Impress enhances the intermediate vision of monofocal patients.
It provides the same best-corrected mean distance acuity as a standard monofocal aspheric IOL.
|
Model name |
XY1A-SP |
|
Optic design |
Biconvex with square, thin and textured optic edge |
|
Optic material |
Hydrophobic acrylic Vivinex™ with UV- and blue light filter |
|
Haptic design |
Textured-rough haptic surface |
|
Diameter (optic/OAL) |
6.00 mm / 13.00 mm |
|
IOL Power (Spherical Equivalent) |
+6.00 D to +30.00 D in increments of 0.50 D
|
|
Injector |
multiSert™ preloaded |
|
Front injector tip outer diameter |
1.70mm |
|
Recommended incision size |
2.20mm |
|
Nominal A-constant * |
118.8 |
|
Optimized constants ** |
Haigis a0 = -1.0459 a1 = 0.2547 a2 = 0.2291 Hoffer Q pACD = 5.700 Holladay 1 sf = 1.928 SRK/T A = 119.193 |

Introducing Vivinex™ Gemetric™ & Vivinex™ Gemetric™ Plus
Our trifocal family of IOLs designed to provide a full range of vision
VivinexTM GemetricTM – Is designed to provide excellent distance vision and well balanced intermediate and near vison.
VivinexTM GemetricTM Plus – Is designed to provide excellent near vision and well balanced distance and intermediate vision.

