Glaukos Corporation is an ophthalmic medical technology and pharmaceutical company focused on the development and commercialization of novel surgical devices and sustained pharmaceutical therapies designed to transform the treatment of glaucoma, one of the world’s leading causes of blindness.
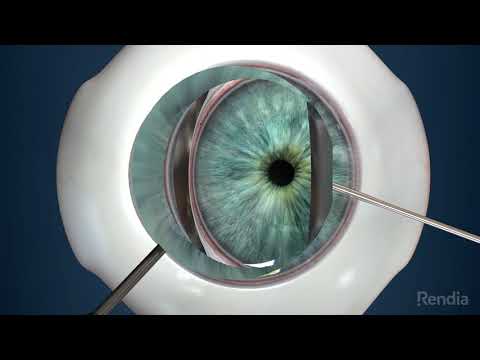
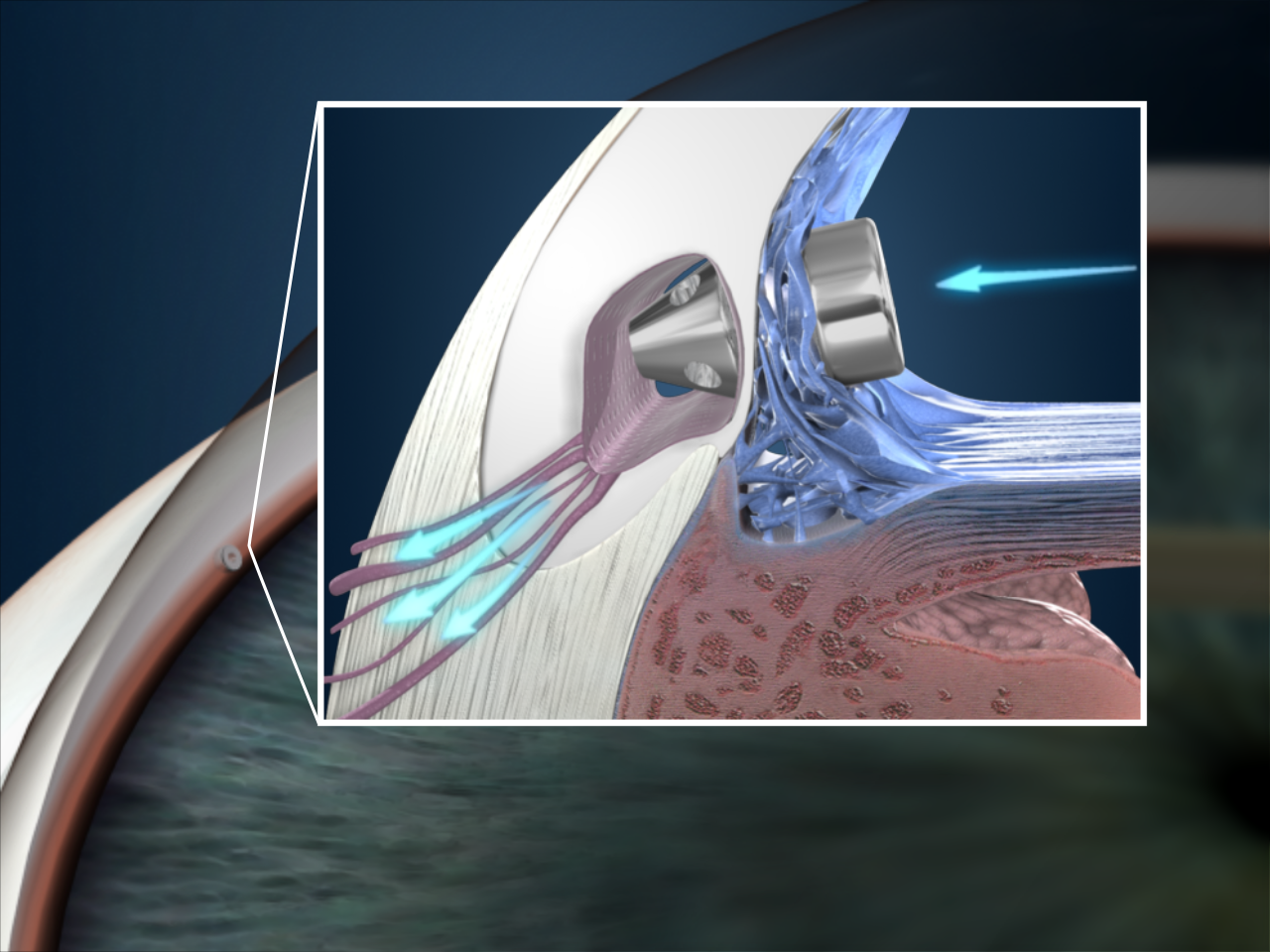
The company pioneered Micro-Invasive Glaucoma Surgery, or MIGS, in order to revolutionize the traditional glaucoma treatment and management paradigm. Glaukos launched the iStent®, its first MIGS device, in the United States in 2012 and is leveraging its platform technology to build a comprehensive and proprietary portfolio of micro-scale injectable therapies designed to address the complete range of glaucoma disease states and progression.
The company’s second-generation MIGS device, the iStent inject® Trabecular Micro-Bypass System, was approved by the Food and Drug Administration, or FDA in June 2018. The company believes the iStent inject,® measuring 0.23 mm wide and 0.36 mm long, is the smallest medical device ever approved by the FDA.
In June 2015, Glaukos completed an initial public offering and our shares are now traded on the New York Stock Exchange under the ticker symbol “GKOS”. The company was founded in 1998 and is based in San Clemente, California.
iprism ® SX delivers precise lens handling during intraoperative gonioscopy procedures and is the clear choice for optimizing iStent inject ® trabecular micro-bypass procedures – all in a convenient single-use format.
- Small lightweight handle designed to be held in either hand with optimized ergonomics, which delivers outstanding balance and ease of use.
- Innovative biconic optic offers a 1.25 magnified view of angle structures and an overall wide view of the anterior chamber.
- Gentle stabilisation features integrated into the gonioprism centre the view, and provide dependable, steady control.
- Alignment guides enable confident stent placement by optimizing positioning and clock hour spacing.
- Single-use, sterile device, conveniently packaged and ready for use, with no need for cleaning, sterilisation, or disinfection.

The newly designed iprism® S provides exceptional clarity with expanded views of all angle structures. Iprism S offers new design features for precise lens handling during intraoperative gonioscopy procedures.
Unique Features:
- Expanded incision access eliminates device interference at the incision during gonioscopy
- Wide angle lens dramatically expands field of view for a broader perspective of all angle structures
- Unique concave lens geometry delivers precise coupling with the corneal surface for a quick, clear, and ”self-centering” view of the trabecular meshwork
- Anti-reflective technology delivers crystal clear views of angle structures without light distortion or interference
- Lightweight, low-profile design fits elegantly in the fingers for unparalleled control and enables lighter touch for reduced corneal striae